Pfizer and Moderna Develop Potential Coronavirus Vaccines
November 20, 2020
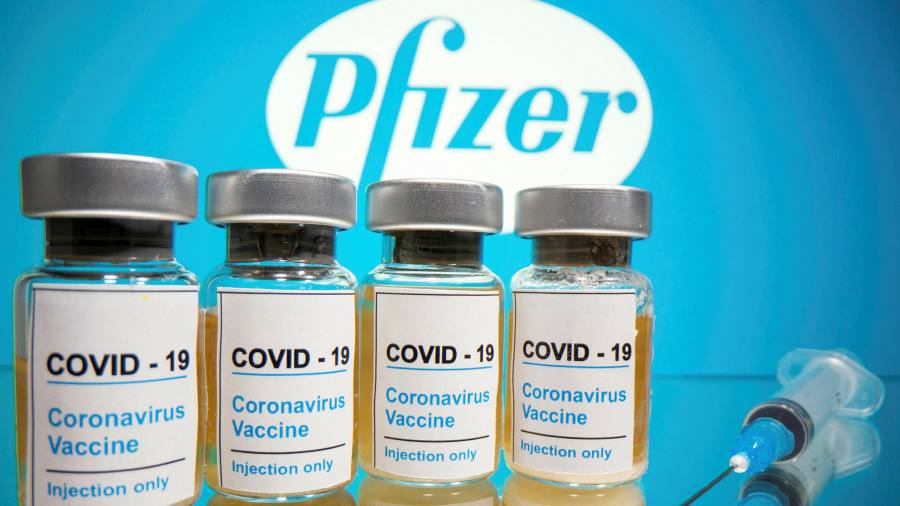
Last week, Pfizer announced their coronavirus vaccine candidate was up to 90% effective, and on November 16th, Moderna declared their vaccine 94.5% effective.
Both companies will be trying to get emergency authorization from the FDA for these vaccines within weeks, as their testing has greatly exceeded the FDA’s requirement of the vaccine being at least 50% effective. According to officials, both vaccines could supply 20 million Americans during December with priority being given to essential workers and those most at-risk to the virus.
“If the vaccine passes all the tests and has a high success rate, I would get the vaccine,” comments Senior Eddie Lynch.
Pfizer’s vaccine requires extremely cold temperatures to be stored and shipped at a low of -70 degrees Celsius, while Moderna’s needs to be stored at -20 degrees Celsius. This low temperature is significantly below the standard for vaccines of 2 to 8 degrees Celsius. Pfizer has developed special deep freeze suitcases that are tightly sealed for shipping the vaccine, but these difficult conditions will be a problem for storage in hospitals and pharmacies. Shipments to the US for the Pfizer vaccine have already started, and they chose Rhode Island, Texas, New Mexico, and Tennessee as its starters due to their diversity in populations and differences in size along with rural and urban settings.
Moderna shocked the world by announcing their vaccine was 94.5% effective. Moderna conducted a study where they randomly assigned either the vaccine or a placebo to 30,000 people. Out of the first 95 people to contract the coronavirus, only 5 of these patients were recipients of the treatment rather than the placebo. While these results may be great news, the vaccine will most likely not be ready for widespread distribution until the spring of 2021 at the earliest.
These potential vaccines have a special approach to activate the body’s immune defenses using messenger RNA (mRNA), which has never been approved by the FDA in vaccines before. Messenger RNA enables cells to create a particular coronavirus protein that produces an immune response. Thus, the vaccine cannot infect someone with COVID-19 but provides the body’s immune system with the ability to fight the virus more easily once infected.
“I was really excited when I heard the news about the vaccines. I cannot wait for this pandemic to be over and for life to go back to normal,” said Senior Jack Ziegler.
While Pfizer and Moderna may be the frontrunners, there are strong efforts for a potential vaccine in Australia, Great Britain, China, India, Russia, and at least 50 other candidates in the earlier stages of testing.