Oxford University Takes the Lead in Producing Coronavirus Vaccine
May 12, 2020
While the death toll continues to rise due to Coronavirus complications and state officials look to begin the reopening process, companies race to establish an effective vaccine during the current health crisis, of which Oxford University has quickly taken to lead.
As the global pressure to create a COVID-19 vaccine intensifies, according to Desert News, there are over 100 different candidates for a possible vaccine in the works, some more promising than others. There are currently eight undergoing the human trial phase.
Amidst the chaos, Oxford University Medical Center, a prestigious institution in England, has already established a solid timeline of development and production measures.
Their team, led by professor of vaccinology at Jenner Institute Sarah Gilbert, was able to move swiftly, as they had already developed a vaccine for Middle East Respiratory Syndrome (MERS), a virus very similar to COVID-19.
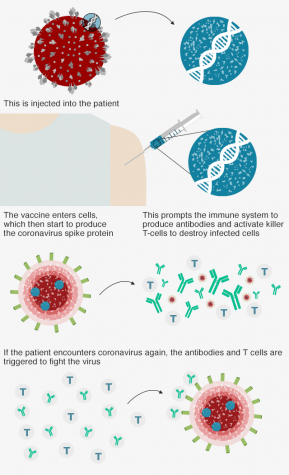
To create the vaccine, dubbed ChAdOx1 nCoV-19, the Oxford team first manipulated a version of the adenovirus, which causes the common cold virus in chimpanzees.
Then, this adenovirus was further modified to carry genetic material from SARS-CoV-2, which codes for the spike-like protrusions on the surface of the Coronavirus molecule.
These spikes in the exterior of the virus are the point of contact that invade and harm human lung cells.
Throughout this process, the vaccine was also altered to ensure that it will not harmfully thrive in the human body, but to serve as a precursor for the immune system to begin producing antibodies if the COVID-19 virus was to invade.
In other words, when injected, the vaccine allows the immune system to identify the proteins that build the Coronavirus, preparing the body to spring into action if COVID-19 was to enter the body.
The vaccine has shown promising early results. Six animals that were given the vaccine then exposed to the virus did not contract the virus after being monitored for 28 days.
Within the human trials in the UK, 800 people volunteered. 400 will receive the ChAdOx1 nCoV-19 vaccine, and the rest will receive a placebo.
The Serum Institute of India, the world’s largest producer of modern vaccines, has already begun to produce the vaccine and could produce up to 60 million doses this year.
If all goes well, Oxford states that it may be released to the public in the UK and globally by September.
Other companies, such as Moderna, based in Cambridge Massachusetts, have begun phase 2 trials and have received much support form the United States government.
However, they predict that they will not be able to begin phase 3 human trials until summer, pushing them about 2 months behind Oxford.
Despite this 2 month lag period, Moderna still hopes to be able to issue tens of millions of vaccines by the end of this year as well.